Transforming Pencil Graphite into Diamonds: A Surprising Science
Written on
Chapter 1: The Unexpected Connection Between Graphite and Diamonds
Could it be possible that every pencil lead hides a diamond at its tip? It seems far-fetched, but the truth is more connected than you might think.

Photograph by Yoann Siloine on Unsplash
Diamonds are typically assessed based on the “four Cs”: Carat, Clarity, Color, and Cut. If every pencil contained diamonds, the world would surely take notice. The surprising fact is that between graphite, the material in pencil leads, and diamonds, the differences are not as vast as they appear.
A quick glance at the following comparison table illustrates the contrasts:
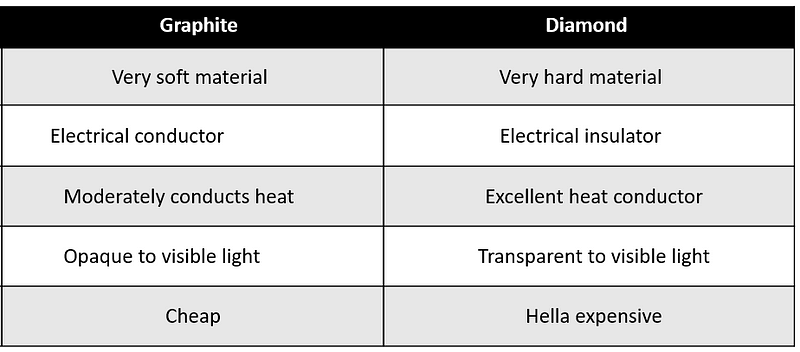
Despite their differences, if we were to anthropomorphize them, we might consider them as kin—sharing the same elemental "blood" of carbon.
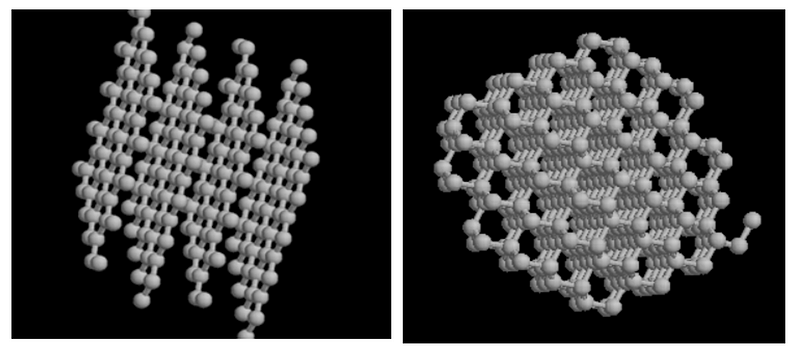
(1) Graphite ; (2) Diamond Diamonds are renowned as the hardest naturally occurring substance, capable of scratching any material while remaining untouchable themselves. Like two siblings who may not look alike, their true similarity lies in their core composition.
The fundamental difference between graphite and diamond arises from the arrangement of their carbon atoms. This arrangement, known as crystalline structure, is key to understanding their distinct physical and chemical properties. In diamonds, carbon atoms form a robust three-dimensional network, with each atom covalently bonded to four others, resulting in their incredible hardness and clarity. Conversely, in graphite, carbon atoms align in flat, hexagonal layers, bonded strongly within the same plane but weakly between layers. This structure grants graphite its soft texture and electrical conductivity.
The stark contrasts reveal that while both graphite and diamond are composed of carbon, their atomic arrangements lead to vastly different properties.
Section 1.1: The Role of Pressure and Temperature in Formation
The formation of diamonds occurs at depths ranging from 140 to 190 kilometers beneath the Earth's surface within the mantle. But why does one structure emerge over the other? The answer lies in the environmental conditions—specifically, temperature and pressure.
The diagram below simplifies this concept:
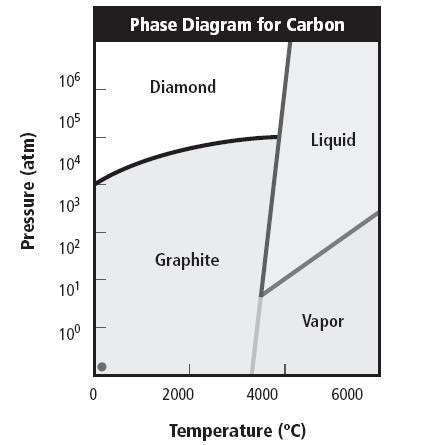
This phase diagram illustrates temperature on the x-axis and pressure on the y-axis. By identifying specific values of temperature and pressure, we can locate a point on the graph. If this point falls within the diamond formation zone, diamonds will develop; if within the graphite zone, graphite will form.
At extremely high temperatures, the material transitions to a vapor or liquid state, precluding solidification.
Section 1.2: From Graphite to Diamonds: A Feasible Transformation
Graphite, commonly found in pencils, is also utilized in lubricants and batteries. Interestingly, around 80% of mined diamonds are repurposed for industrial use, particularly as abrasives.
So, can we alter graphite to become diamond and vice versa? While diamonds in stores exist under low-pressure and temperature conditions, stabilizing their structure post-formation, the transition of graphite to diamond is indeed achievable in laboratory settings through precise manipulation of temperature and pressure.
Conversely, converting diamonds back to graphite necessitates heating the diamond to extremely high temperatures in an oxygen-free environment to prevent combustion.
Final Thoughts: Quick Recap
- Carbon Structure: Both diamond and graphite consist of carbon but differ in their atomic arrangements; diamonds have a 3D network of covalently bonded atoms, while graphite’s layers allow for sliding.
- Formation Conditions: Diamonds develop deep within the Earth’s mantle under high pressures and temperatures, while graphite forms under significantly milder conditions.
- Physical Properties: These variations in atomic structure and bonding lead to unique characteristics, such as diamond's remarkable hardness.
- Conversion Potential: Natural conditions dictate whether carbon becomes graphite or diamond, but in controlled laboratory environments, graphite can be transformed into diamond.
Do you believe mined and synthetic diamonds hold the same value? Thank you for engaging with this exploration of carbon's fascinating forms! Looking forward to our next discussion.
Related Articles: - The World’s Rarest Gemstone - The World’s Deepest Mine
Explore more on Geology & Earth Sciences Learn about the forces shaping our planet and the dynamic processes occurring underground, at sea, and in the atmosphere.
Follow us on X (Twitter), TikTok, and Instagram.