Breakthrough Gene Therapy Offers Hope for Rare Genetic Disorders
Written on
Chapter 1: Introduction to AADC Deficiency
A significant advancement in gene therapy has occurred with a Phase 1 trial conducted on children afflicted by an extremely rare genetic disorder known as AADC deficiency. This groundbreaking therapy, inspired by previous research on Parkinson’s disease, has yielded remarkable outcomes.
The AADC deficiency, which affects only 135 children worldwide, is characterized by the absence of the AADC enzyme. This disorder disproportionately impacts individuals of Asian descent, leading to severe challenges in basic functions such as speaking, feeding, and maintaining head control.
The lead researcher, Krystof Bankiewicz, previously developed gene therapy for Parkinson’s patients, leading the team to explore its potential application in children suffering from AADC deficiency. The results of this endeavor have been remarkably promising.
Section 1.1: Study Details
In the clinical trial, seven children aged between 4 and 9 received the gene therapy treatment. Six participants were treated at UCSF, while one was treated at Ohio State Wexner Medical Center.
Subsection 1.1.1: Initial Outcomes
According to Krystof Bankiewicz, "In the months that followed, many patients experienced life-changing improvements. Not only did they begin laughing and have improved mood, but some were able to start speaking and even walking."

The results were profound; nearly all participants, except one, experienced a reduction in seizures and demonstrated enhanced motor function, mood, and sleep quality. These improvements allowed them to engage more effectively with their families.
This video highlights the incredible journey of a young boy with a rare disease who begins innovative gene therapy.
Section 1.2: Methodology of Treatment
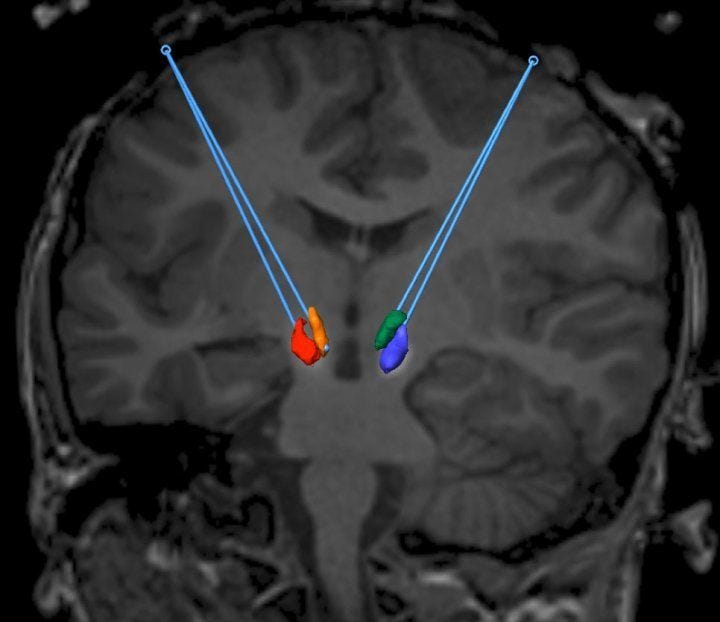
The treatment's approach diverged from earlier methods used for Parkinson’s disease, targeting two specific midbrain regions rich in dopamine-producing neurons: the substantia nigra pars compacta and the ventral tegmental area.
A viral vector containing the AADC gene was created, and using real-time Magnetic Resonance Imaging (MRI), this vector was infused into the brain through a small opening in the skull. This method proved more manageable than treatments for Parkinson’s, as the neuronal pathways remain intact; the primary issue is the inability to produce dopamine due to the lack of AADC.
Chapter 2: Results and Future Directions
Post-treatment imaging revealed increased AADC activity in the brain, alongside higher dopamine levels. Symptoms such as irrita